
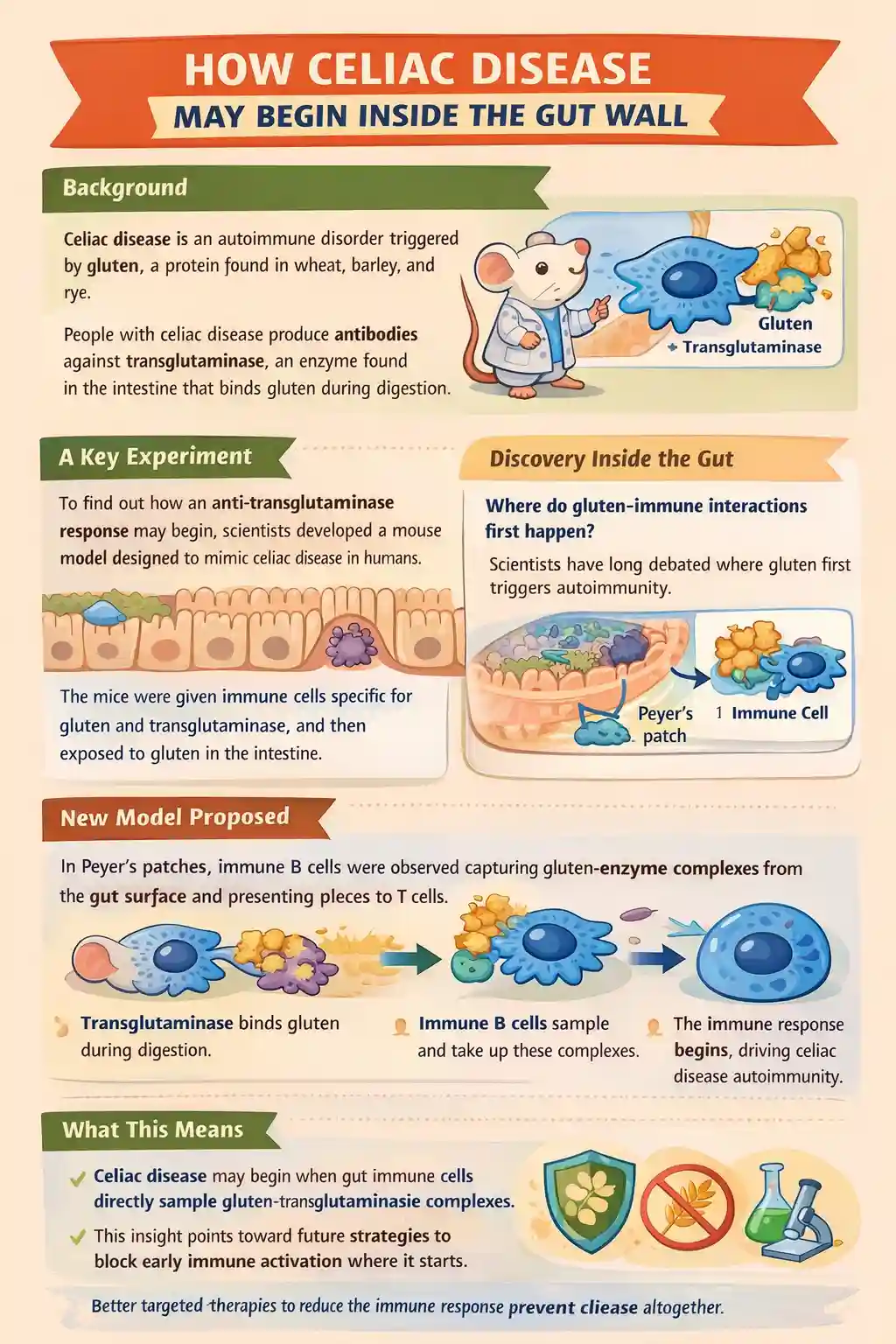
Celiac.com 01/27/2026 - This study set out to answer a long-standing question in celiac disease research: where and how the immune system first becomes activated against gluten and the body’s own enzyme, transglutaminase. People with celiac disease produce antibodies against transglutaminase, even though it is a normal human protein. Scientists have known for years that this autoimmune response somehow depends on gluten, but the exact location and sequence of events that link gluten exposure to autoimmunity have remained unclear. This research uses a carefully designed mouse model to show that key immune interactions may begin directly within specialized immune structures in the gut wall called Peyer’s patches.
Background: Why Transglutaminase and Gluten Are Linked
Celiac disease is unusual among autoimmune disorders because the target of the immune response is a normal enzyme that is present throughout the body. Transglutaminase modifies gluten proteins during digestion, and this chemical interaction appears to create a bridge between gluten and the immune system. Immune cells that recognize gluten provide help to other immune cells that specifically recognize transglutaminase. This cooperation leads to the production of antibodies against transglutaminase, which are a defining feature of celiac disease and are widely used for diagnosis.
Celiac.com Sponsor (A12):
Despite this understanding, researchers have struggled to explain where transglutaminase and gluten first meet in a way that triggers immunity. One possibility is that this interaction happens deep in the intestinal tissue after gluten has crossed the gut barrier. Another possibility is that the interaction begins much earlier, closer to the gut surface, within immune structures that actively sample material from the intestinal contents.
Purpose of the Study
The main goal of this study was to determine whether immune cells located in gut-associated lymphoid tissues can directly encounter complexes formed between transglutaminase and gluten. Specifically, the researchers wanted to test whether B cells that recognize transglutaminase can capture this enzyme from the gut lumen and, with help from gluten-reactive T cells, initiate the autoimmune response characteristic of celiac disease.
To address this, the researchers developed a specialized mouse model that reproduces essential features of human celiac disease, including genetic susceptibility and the presence of gluten-reactive immune cells.
How the Mouse Model Was Designed
The researchers used mice that were genetically engineered to express a human immune molecule strongly associated with celiac disease. These mice were given two specific types of immune cells: B cells that recognize transglutaminase and T cells that recognize gluten. This setup allowed the investigators to observe how these cells interact after gluten exposure.
The mice were then fed a specially designed protein that mimics the interaction between transglutaminase and gluten. This protein included parts that could be recognized by both B cells and T cells, ensuring that cooperation between these immune cells could occur. An immune-stimulating agent was included to encourage responses in the gut, allowing the researchers to closely track immune activation in intestinal tissues.
Development of a Celiac-Like Immune Response
After oral exposure, the mice developed immune responses that closely resembled those seen in people with celiac disease. Antibodies directed against transglutaminase appeared both in the bloodstream and in the gut. In the intestinal lining, immune cells that produce these antibodies were detected in locations similar to those seen in untreated human disease.
This confirmed that the model successfully reproduced key features of celiac autoimmunity, making it possible to study where and how the immune response begins.
The Role of Peyer’s Patches
A major focus of the study was Peyer’s patches, which are organized immune structures embedded in the wall of the small intestine. These structures are strategically positioned to sample material from the gut lumen and initiate immune responses when necessary.
The researchers found that transglutaminase-specific B cells became activated and expanded within Peyer’s patches after oral exposure to the model antigen. These B cells showed features of active immune participation, including characteristics associated with antibody refinement and long-term immune memory. At the same time, gluten-reactive T cells in the same locations showed signs of providing help to these B cells, consistent with cooperative immune activation.
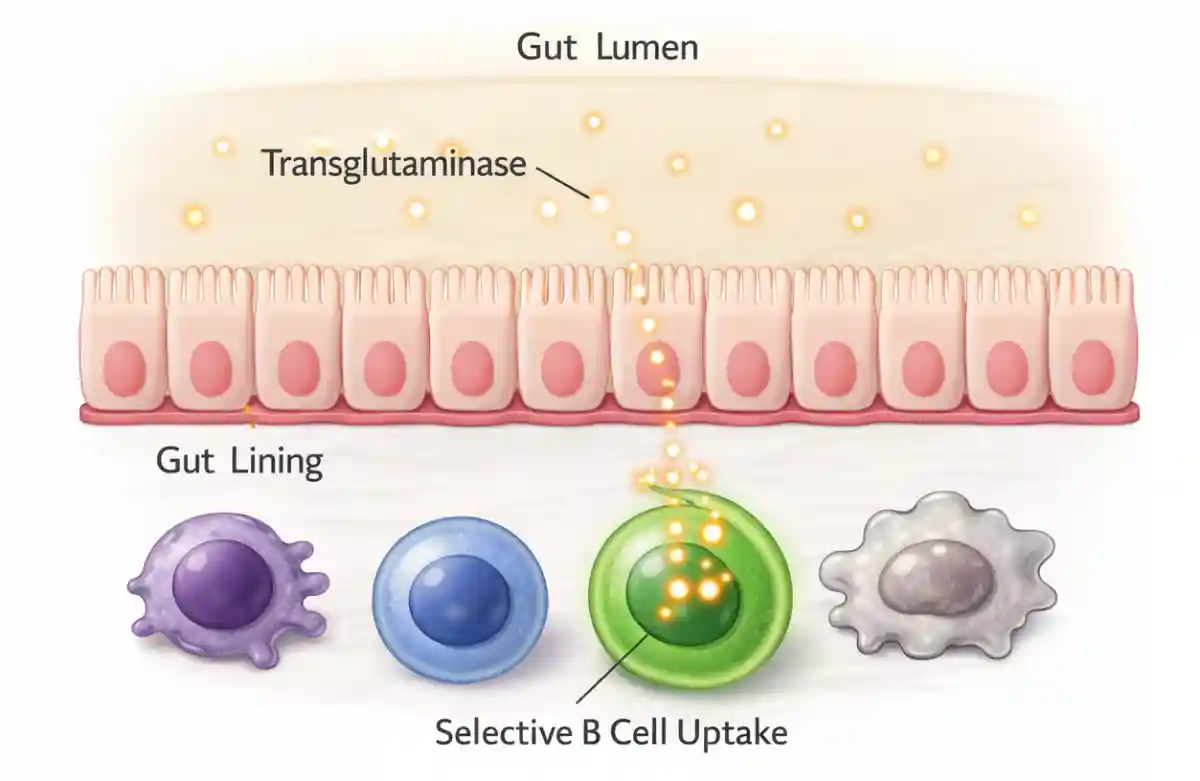
Direct Sampling of Transglutaminase From the Gut Lumen
One of the most important experiments in the study tested whether B cells in Peyer’s patches can directly sample transglutaminase from inside the gut. To do this, the researchers introduced labeled transglutaminase into a section of the intestine that contained Peyer’s patches and then examined the tissue using high-resolution imaging.
They observed that B cells specific for transglutaminase captured the enzyme from the gut lumen. This uptake occurred in precise regions of Peyer’s patches that are known to specialize in antigen sampling. Importantly, this process was selective: only B cells that recognized transglutaminase took up the enzyme, while other immune cells did not.
This finding provides direct evidence that transglutaminase present in the gut lumen can be captured by immune cells without needing to first cross the intestinal barrier in a non-specific way.
A New Model for How Celiac Disease Begins
Based on these results, the researchers propose a model in which transglutaminase binds gluten in the gut lumen during digestion. These enzyme-protein complexes are then sampled by specialized B cells in Peyer’s patches. Once captured, the B cells present gluten fragments to gluten-reactive T cells, receiving help that drives the production of antibodies against transglutaminase.
This sequence of events offers a coherent explanation for how dietary gluten leads to a highly specific autoimmune response. It also suggests that the earliest steps of celiac disease may occur at the gut surface rather than deeper within intestinal tissue.
What the Study Does and Does Not Show
While the findings are compelling, the study does not directly demonstrate the uptake of naturally occurring transglutaminase-gluten complexes formed during normal digestion. Instead, it shows that transglutaminase itself can be sampled from the gut lumen by specific immune cells. The researchers also note that other immune cells may contribute to antigen presentation and that further work is needed to fully map all pathways involved.
Even so, the model provides a powerful experimental platform for studying early immune events that are difficult or impossible to observe directly in humans.
Why This Study Matters for People With Celiac Disease
For people with celiac disease, this research offers a clearer picture of how gluten exposure can rapidly lead to immune activation. It supports the idea that the disease may begin within the gut wall itself, without requiring a generalized breakdown of the intestinal barrier. This challenges older theories that focused on widespread gut leakiness as the primary trigger.
Understanding that immune activation may start with specific interactions in Peyer’s patches opens new possibilities for treatment. Therapies could be designed to block the formation or uptake of transglutaminase-gluten complexes, interfere with early B cell and T cell cooperation, or target transglutaminase activity in the gut lumen. Such approaches could one day complement a gluten-free diet or reduce the immune consequences of accidental gluten exposure.
In summary, this study provides strong evidence that the roots of celiac disease lie in carefully orchestrated immune sampling events within the gut wall. By identifying where and how the autoimmune response begins, it moves the field closer to more precise and potentially transformative therapies for people living with celiac disease.
Read more at: gastrojournal.org and medscape.com








Recommended Comments
There are no comments to display.
Create an account or sign in to comment
You need to be a member in order to leave a comment
Create an account
Sign up for a new account in our community. It's easy!
Register a new accountSign in
Already have an account? Sign in here.
Sign In Now