Celiac.com 12/19/2025 - This study explores the relationship between two autoimmune conditions—celiac disease and autoimmune gastritis. While celiac disease affects the small intestine and is triggered by gluten, autoimmune gastritis targets the stomach lining. Both can lead to nutrient deficiencies and anemia, making their coexistence a serious health concern. The research aimed to find out how common autoimmune gastritis is among people with celiac disease and to identify which factors might help predict who is most at risk.
Background: Two Autoimmune Conditions with Overlapping Effects
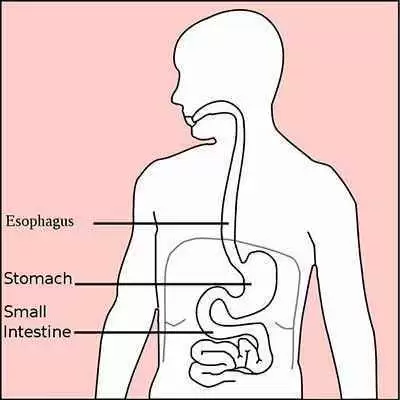
Celiac disease occurs when the body’s immune system reacts to gluten, a protein found in wheat, barley, and rye. This reaction damages the inner lining of the small intestine, preventing proper absorption of nutrients such as iron, calcium, and vitamins. Symptoms vary widely—from digestive problems to fatigue, skin issues, and neurological complaints—but the only effective treatment is a strict lifelong gluten-free diet.
Celiac.com Sponsor (A12):
Autoimmune gastritis, on the other hand, is a disorder in which the immune system mistakenly attacks the stomach’s own cells, particularly those responsible for producing acid and intrinsic factor—a substance needed to absorb vitamin B12. Over time, this attack leads to thinning of the stomach lining, reduced acid production, and impaired nutrient absorption. Like celiac disease, autoimmune gastritis may cause iron deficiency, vitamin B12 deficiency, and eventually anemia.
Because both diseases are immune-related and can cause similar nutrient problems, researchers have long suspected they might occur together more often than expected. This study set out to measure that connection and identify key indicators that could help doctors detect both conditions early.
Study Design and Purpose
The research involved 183 patients who had already been diagnosed with celiac disease. Each participant’s data were carefully reviewed to determine whether they also showed evidence of autoimmune gastritis. Researchers collected information on age, gender, other autoimmune conditions, antibody test results, and nutrient levels.
Celiac disease diagnoses were classified using the Marsh system, which describes how severely the small intestine has been damaged by gluten exposure. Autoimmune gastritis was confirmed using tissue samples from the stomach, analyzed under a microscope for characteristic changes.
The main goals were to find out how many people with celiac disease also had autoimmune gastritis, and to determine whether any clinical or laboratory findings could predict which patients were more likely to have both diseases.
What the Researchers Found
Among the 183 people with celiac disease, 19 were also diagnosed with autoimmune gastritis. This means that approximately 10 percent of the study participants had both conditions. This figure is higher than what would normally be seen in the general population, suggesting a meaningful overlap between the two diseases.
When the researchers analyzed different factors that might explain this connection, two stood out as statistically significant:
- Marsh Type 2 Celiac Disease: Patients with this intermediate level of intestinal damage were much more likely to also have autoimmune gastritis. The odds were more than 15 times higher compared to patients with other forms of celiac disease.
- Absence of Anti-Endomysial IgA Antibodies: These antibodies are commonly found in people with active celiac disease and are used to confirm diagnosis. Interestingly, patients who did not have these antibodies were more likely to have autoimmune gastritis.
These findings suggest that a specific subset of celiac patients—those with certain intestinal changes and lacking typical antibodies—might be more vulnerable to developing autoimmune gastritis.
Why These Findings Matter
Autoimmune gastritis often goes unnoticed because it can progress slowly and cause vague symptoms such as fatigue, heartburn, or mild abdominal discomfort. However, if left untreated, it can result in serious nutrient deficiencies, particularly vitamin B12 and iron, which can cause anemia, nerve damage, and long-term health complications.
For people with celiac disease, these same deficiencies can also occur from intestinal damage. When both diseases occur together, the risk of nutrient imbalance increases dramatically. Identifying patients who may have both conditions allows doctors to monitor them more closely, correct deficiencies early, and reduce complications such as fatigue, dizziness, and neurological problems.
Screening Recommendations
Based on the study’s findings, the researchers recommend that doctors consider screening for autoimmune gastritis in specific groups of celiac patients—particularly those who:
- Have Marsh type 2 intestinal changes (moderate inflammation and damage).
- Do not test positive for anti-endomysial IgA antibodies, despite confirmed celiac disease.
Screening may involve blood tests to detect stomach-specific antibodies and, if necessary, an upper endoscopy with biopsy to confirm inflammation and structural changes in the stomach lining. Detecting autoimmune gastritis early allows for proper treatment of vitamin and iron deficiencies and helps prevent long-term complications.
Connecting the Dots: Shared Autoimmune Roots
Both celiac disease and autoimmune gastritis are driven by an overactive immune response that mistakenly targets the body’s own tissues. In celiac disease, gluten exposure triggers this reaction in the small intestine; in autoimmune gastritis, the immune system attacks stomach cells responsible for digestion and nutrient absorption.
The presence of both conditions in the same person may reflect a broader tendency toward autoimmune reactivity. It also highlights the importance of a holistic approach to managing autoimmune conditions—once one such disease is diagnosed, others should be considered and screened for.
Implications for People with Celiac Disease
For people already living with celiac disease, this study provides valuable insight into potential hidden complications. Even after starting a gluten-free diet, some patients continue to experience fatigue, anemia, or lingering digestive symptoms. In these cases, autoimmune gastritis could be a contributing factor. Recognizing and treating it can restore energy, improve nutrient absorption, and enhance overall well-being.
Additionally, understanding this connection can help patients and healthcare providers take a proactive approach. Regular blood work to monitor iron, vitamin B12, and folate levels can help catch problems early. A multidisciplinary care team—including gastroenterologists, dietitians, and immunologists—can ensure that all aspects of health are managed effectively.
Conclusion
This study demonstrates that autoimmune gastritis affects roughly one in ten people with celiac disease. Those with moderate intestinal changes (Marsh type 2) and without typical celiac antibodies appear to be at the greatest risk. Because both conditions can cause nutrient deficiencies and anemia, identifying and treating autoimmune gastritis in celiac patients is essential for long-term health.
For people with celiac disease, these findings highlight the importance of ongoing medical follow-up—even after switching to a gluten-free diet. Early detection and management of coexisting autoimmune conditions can prevent serious complications and improve quality of life.
Read more at: researchgate.net









Recommended Comments
There are no comments to display.
Create an account or sign in to comment
You need to be a member in order to leave a comment
Create an account
Sign up for a new account in our community. It's easy!
Register a new accountSign in
Already have an account? Sign in here.
Sign In Now